With any risk assessment, the first step is the identification of the hazard. In many respects, and especially when dealing with dangerous substances like liquids and gases, we are given a clue as to what the hazard is because it comes with hazard pictograms on the container.
Below are examples of toxic and flammable hazard pictograms.

Flammable Hazard Pictogram

Toxic Hazard Pictogram
When dealing with dangerous substances like gasoline or acetone, both the container and the Material Safety Data Sheet (MSDS) will supply information on the best way to manage that material and its properties. The MSDS will also provide information regarding the flammable conditions and flammable properties of the material.
Combustible Dust and the MSDS
For combustible powders, on the other hand, MSDS information is rarely given. There are no original pictograms that can be stuck on powder solids that have the potential to combust and create a dust explosion.
This is problematic for the creation of suitable process safety mitigation measures that protect the health and safety of employees in the workplace, even though combustible powder solids are common both in the workplace and in our daily lives.
Common kitchen items such as flour, sugar and icing sugar have the potential to combust and can readily combust. When we buy a bag of flour or icing sugar, for example, the packaging does not mention that it is a combustible solid.
In industry, a Material Safety Data Sheet will be provided, but often, it does not provide adequate information. Often, it will simply state that the material may be capable of combusting. Without understanding the specific properties of a material, it is difficult to use that data to effectively prevent fires and explosions.
What is Combustible Dust?
Any substance that has the potential to become dispersed and suspended through the air as a dust cloud, ignite, and explode when exposed to an ignition source is considered to be combustible dust and may create an explosion hazard.
Materials that are in the physical states of powders, flakes, fines, fibres, etc. may be included in combustible dust. Most solid organics (e.g. flour, grain, and wood), carbonaceous (charcoal, soot, coal), textile fibres (cotton), metals, and some non-metallic inorganic elements are all examples of combustible dusts.
Those that may not “normally” be combustible, can burn or explode if the dust particles are at the right size and concentration.
When mixed with oxygen, these fine particles can ignite when coming into contact with an ignition source i.e. a spark, or metal ember. The propensity of the hazard is primarily associated with the particle size distribution or fineness of the powder – this dust material can be dispersed in air readily creating an explosive atmosphere.
Such flammable atmospheres may occur as they are sensitive to ignition either by sparks (electrostatic or mechanical) or hot surfaces.
ATEX & DSEAR: Combustible Dust Legislation
As expected, there is legislation on the topic of combustible dust and safety. There is the ATEX/DSEAR legislation in Europe and the UK, similar legislation in the US, and elsewhere around the world. But all this legislation provides three simple questions.
Avoiding a Flammable Atmosphere
Avoid generating what is known as a flammable or explosive atmosphere. Explosive atmospheres are a mixture of fuel and oxygen that are capable of combustion. This is simply built around what is known as the fire triangle. For combustion to occur and for something to burst into flame, there needs to be fuel alongside air or oxygen and an ignition source.
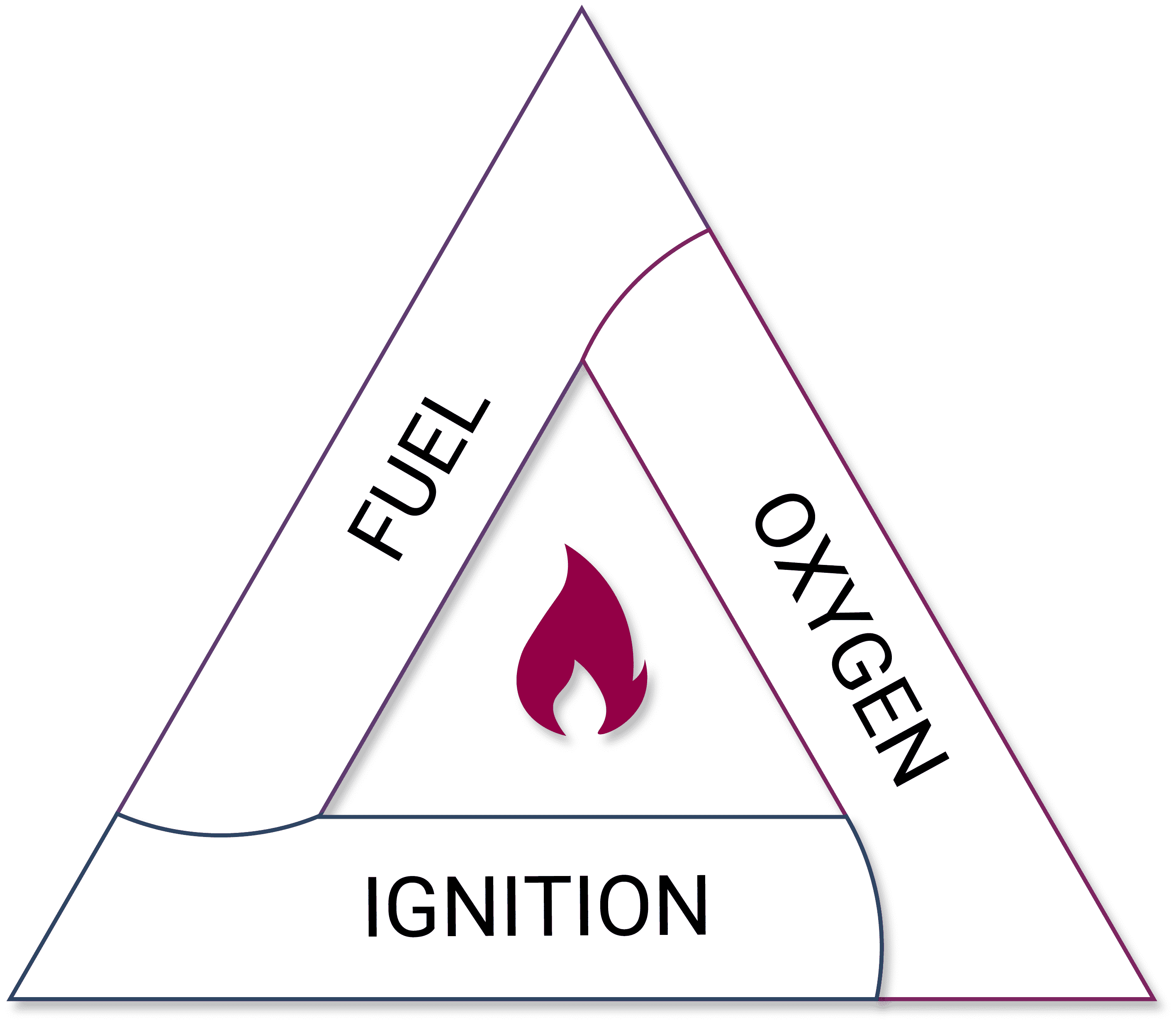
Fire Triangle
Take, for example, a flammable gas like methane. When it is contained in a pipe it is 100% methane, but as there is no air in the pipes, it is not flammable. The container will state that it is an extremely flammable gas, but in practice, it will not ignite because there is insufficient air.
If there is a leak from that system and methane is released into the general atmosphere, it will produce a flammable explosive atmosphere as it is mixed with air.
So, the first point is can we avoid generating that flammable explosive atmosphere?
Avoiding Ignition in Explosive Atmospheres
Secondly, if we cannot avoid generating a flammable atmosphere, because there is the potential for containment systems to leak, then do not ignite this atmosphere.
There is a lot of legislation that is focused on trying to prevent ignition sources by having ATEX certified electrical and mechanical equipment. These pieces of equipment have been specially designed to not produce an effective ignition source if it is present within one of the flammable or explosive atmospheres.
Avoid Injury or Worse…
Finally, if an explosive atmosphere does ignite (because after all, we are working in a land of probabilities) do not harm anyone. We can do our best to prevent the generation of a flammable atmosphere. We can do our best to prevent an effective ignition source, but it could still happen. So, if it does happen then try it and ensure that nobody is hurt by that ignition.
The Explosion Pentagon
To turn the fire triangle into a potential explosion, two other elements need to be added. If we add into the fire triangle, dispersion, (a good mixing of the oxidant and the fuel). And once there is confinement that restricts the spread of the flame then we can generate an explosion over pressure. This, in essence, is the explosion pentagon.
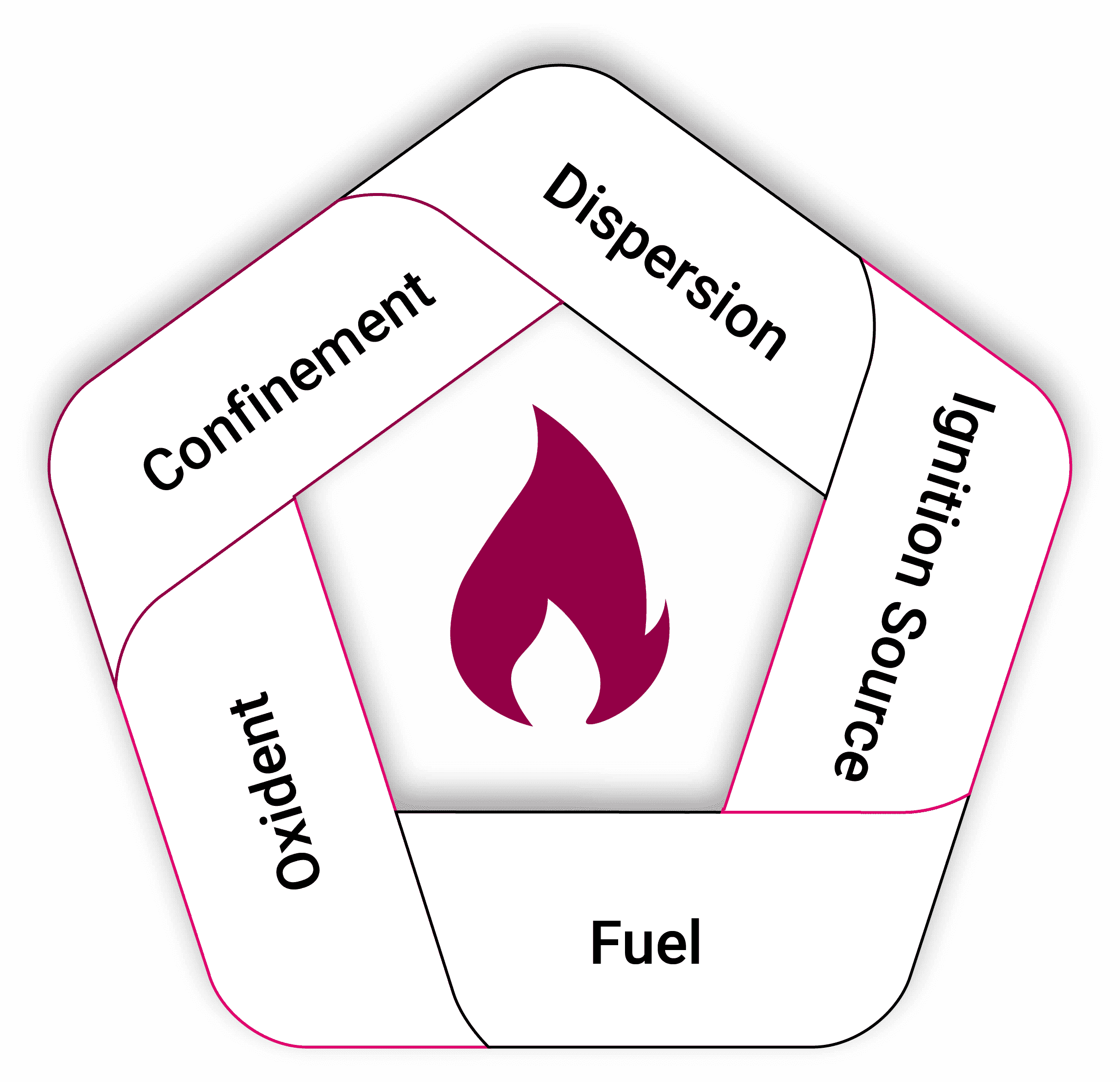
When mixing fuel with air in the open, we can still have mixing but if the confinement element is not there, we will end up with a flash fire. If this occurs within a contained area, like a silo or a pipe, then pressure can be developed and then transitioned into an explosion rather than a fire.
Materials, Chemical Reactions and Combustion
What is a combustion reaction? It is nothing more than a chemical reaction, a material mixing with oxygen. If a material is not fully oxidized then, in theory, it is capable of combusting, so therefore it can react with oxygen. This will be an exothermic reaction that will generate heat which could result in flames.
Overall, liquids and solids do not combust, it is the vapours and gasses that do. As we heat substances, they can generate vapours and these vapours mix with air and then undergo a chemical reaction. So, combustion is just an oxidizing chemical reaction.
Solid Materials and Surface Area
There are many, many substances out there in the food and pharmaceutical industries, for example, that may not be classified as flammable or combustible, but they can and will combust.
Where solids are concerned, the reaction is no different, but it becomes a solid reaction because of surface reaction. Oxygen needs to react with the powder solid, and it can only do that by starting surface chemistry. The surface area-to-volume relationship is important.
The larger the surface area, the easier it is to achieve a reaction and the quicker the reaction will occur. As a result, potential dust explosions due to the above reaction are very dependent on the particle size distribution of that powder. This is the foundation for combustible dust and powders.
As a result of the surface area to volume relationship, it is the fineness of a powder or dust that determines whether it is going to be combustible. And generally speaking, we need to have materials that are finer than 500 microns for them to be considered combustible dust or powders.
Dust Explosion Tests: Dust Combustibility Test
To figure out whether a powder is combustible, there is a simple test known as the dust combustibility test.
A small amount of potentially combustible material is placed on the base of a machine that blows a pulse of air through the substance. This creates a dust cloud/flammable atmosphere, before large electrostatic sparks via electrodes are passed through to see if the material ignites.
If it does ignite, then it is combustible. If it does not, then it is not combustible.
Test Preparation
As with all tests, the potentially combustible solids are prepared and tested to specific standards that call for the material to be sieved. All materials are consequently sieved to less than 500 microns.
The question, therefore, is to ask if the combustible dust can be separated from the bulk material within the process. If the powder itself is a mixture of quite large particles with a little bit of dust, it is likely to not be combustible. Ultimately, if the material is put through this test as an ‘as-is’ sample, it will not combust because there is insufficient combustible dust for a reaction to occur. So, the larger the particles the less likely it is to result in this test returning a combustible result.
Suppliers and Material Data
In many cases, when powders are supplied with supplier information, it is told that the material is not combustible. However, when it is put through a dust combustibility test, it turns out to be combustible.
It can only be assumed that this occurs because the suppliers have undertaken a combustibility test with the material in its ‘as-is’ form. The test has not been conducted correctly according to the standards as they have not sieved out the fine material that can create combustible dust.
Material Examples: Iron Bar
Take a pure iron bar as an example. The material is not combustible, it can melt in furnaces, and it can be manipulated and shaped. When heated up further, it will turn into molten iron. But it will not burst into flame. It is not combustible.
But if that same iron bar were to be ground down to produce iron filings and if those iron fillings were to be tipped into a Bunsen burner flame, it would start to combust that material. If the iron filings were to be ground down even further, then they will turn into a spontaneously combustible material when as soon as it meets air, it will combust.
Again, particle size distribution is important. But there also must be a sufficient ignition source to generate an explosive atmosphere.
Combustible Dust and your Processes
If your processes deal with powders and the processing operations do not necessarily allow for any fine materials to be raised and form an explosive atmosphere via a dust cloud, then that is ok. But there will be cases in a process where attrition can occur.
The processing of certain materials can break that material down into smaller particles. In certain processing operations where dust extraction and collection systems are used, combustible dust and powders are deliberately taken out from the material. Within those unit operations, explosive atmospheres are generated as a result of the very small particle size and the distribution of the material.
Conclusion: Why Test Dust Particles?
It is simple, undertaking a dust combustibility test just provides affirmation regarding whether a process deals with combustible dust and powders or not.
If it is not combustible special precautions will not have to be built into a process because a flammable atmosphere will not be generated.
If it is a combustible dust, then we have got one-third of the fire triangle and one-fifth of the combustion triangle. This will lead to more questions. How much energy is needed to ignite that material? How much confinement is there? What process safety mitigation, containment or suppression systems must be added?



