Chemical reactions are at the core of many processes in the field of chemical manufacturing. These reactions, while designed to yield specific products, often come with inherent hazards in the form of undesired byproducts or heat energy.
These byproducts can manifest as both exothermic and endothermic energy, as well as undesired materials, presenting challenges in maintaining a safe and efficient manufacturing environment.
Among the various hazards associated with chemical reactions, exothermic energy and the generation of permanent gases pose the highest risk potential to any manufacturing process.
The need for a thorough understanding of the process at scale is necessary for both the safety of the personnel involved and the prevention of material and financial loss. Achieving efficiency in manufacturing involves not only optimizing the desired endothermic and exothermic reactions, but also mitigating the risks associated with byproducts in the reaction mixture.
Pressure Emergence: Learning from Industrial Incidents
Runaway reactions, unfortunately, have been implicated in several industrial incidents worldwide. To address this, testing on a small-scale becomes a crucial aspect of process development. The knowledge gained from small-scale testing is then applied to ensure the reliability and safety of reactions conducted on a larger scale.
Pressure emerges as a key hazard in chemical reactions, particularly in vessels. Over-pressurization can lead to either controlled venting or, in extreme cases, explosion.
Like exothermic energy or temperature evolution, pressure fluctuations demand careful consideration due to their significant impact on the overall safety of the process.
Overpressure in a chemical reaction can result from three potential sources:
- Gas Generation: During the normal course of the reaction, the production of gases, either as a product or a byproduct, can contribute to pressure build-up.
- Vapor Pressure Effects: Heat generated or input during the reaction can cause changes in vapor pressure, affecting the overall pressure within the system.
- Heat from the Normal Process: The heat produced during the reaction may trigger secondary reactions, such as decomposition or thermal runaway, leading to the generation of additional gases or an increase in vapor pressure.
A comprehensive approach to chemical reaction hazards involves understanding and managing not only the desired reactions but also the potential risks associated with energy release, byproduct formation, and pressure fluctuations. By prioritizing safety measures and incorporating insights gained from small-scale testing, manufacturers can strive to achieve efficient and secure chemical manufacturing processes.
Pressure Effects: Lessons from Seveso Disaster
Understanding pressure effects, particularly in the context of vapor pressure, is needed to prevent catastrophic events.
Vapor Pressure Dynamics
Imagine a scenario involving a mass of water. As the temperature increases, more vapor is generated above it, a phenomenon intricately tied to the natural logarithmic relationship of vapor pressure. Typically, this relationship between temperature and vapor pressure follows a linear scale, providing a predictable framework for understanding the behaviour of substances under varying thermal conditions.
Graphical Insight: Linear Trends and the Onset of Problems
Examining a graphical representation, the linear correlation between temperature and vapor pressure is evident. The graph, denoted by the green line moving from right to left, illustrates the expected linear increase in temperature. However, the plot takes a significant turn when vapour and gas pressures are combined.
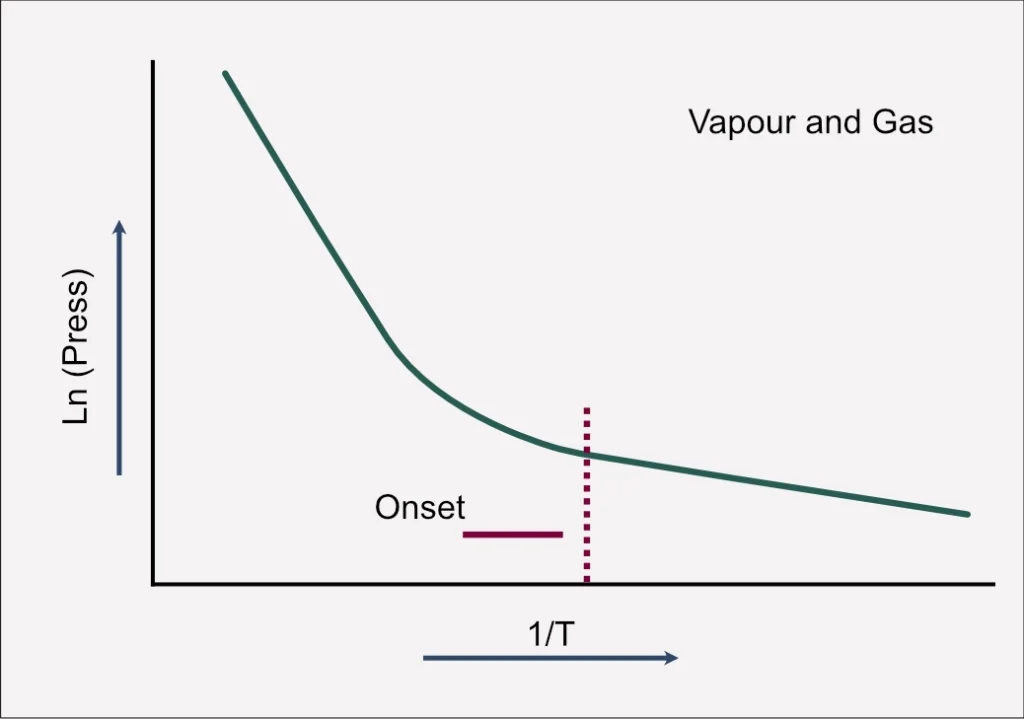
The onset line, a critical juncture in the graph, signifies the commencement of permanent gas generation, possibly from an exothermic reaction. At this point, the trajectory deviates from linearity, spiking upwards. This deviation from the expected linear slope is where challenges emerge, introducing unpredictability and potential risks into the chemical process.
Learning from Seveso
Unfortunately, history provides us with examples of the real-world consequences of inadequate pressure management. The Seveso disaster in 1976 serves as a poignant reminder of the importance of understanding and controlling pressure effects.
In this incident, a thermal runaway event occurred during a standard weekend shutdown. The reactor vessel, employing steam for heating reactions, experienced a lack of stirring due to the shutdown. Localised heating ensued, triggering a thermal runaway reaction whereby more energy is released, that ultimately led to the release of six tons of chemicals into the surrounding area. Despite the activation of the emergency venting system, the escalating pressure, coupled with the absence of stirring, thwarted containment efforts.
While no direct human fatalities were reported, the incident resulted in numerous animal deaths and necessitated a massive cleanup operation.
By understanding the dynamics of vapor pressure and recognising the potential deviations from linearity, professionals in the field can implement targeted measures to mitigate risks and foster a safer, more efficient chemical manufacturing landscape.
Understanding Chemical Reaction Hazard Analysis
The analysis of Chemical Reaction Hazards (CRH) stands as a fundamental step in ensuring both the efficiency, and safety of processes. Understanding why CRH analysis is required and how it integrates into the broader landscape of new methods, developments, and industry practices is essential for navigating the complexities of chemical synthesis.
Early Stages: Identifying New Products and Potential Routes
At the outset of a manufacturing lifecycle, the identification of a new product marks the commencement of the journey. The decision-making process involves exploring potential routes for synthesis, and here, the importance of CRH analysis becomes apparent.
Engaging in early desktop studies is crucial, but equally valuable is the initiation of thermal screening. This involves scrutinising the thermal properties of raw materials or the final products, providing valuable insights that inform decision-making in the nascent stages of production.
Optimising Synthetic Routes: CRH Advantages
As the synthetic route is honed and optimized, the next logical step involves delving into reaction calorimetry. This entails a meticulous examination of the energy released and rate of reactions, offering a comprehensive understanding of the process from both a hazard perspective and an efficiency standpoint. Information gleaned from reaction calorimetry aids in refining the manufacturing process, guiding decisions on factors such as agitation methods and optimal heat points for specific reactions.
Scale-Up Challenges: Necessity of Major Hazard Assessment
The transition from laboratory-scale experimentation to plant-scale production introduces a paradigm shift in the assessment of chemical hazards. Recognising the limitations of lab assessments, particularly in the face of scale differences, becomes crucial. This is where adiabatic calorimetry plays a pivotal role. It forms the bedrock of safety considerations as processes move towards larger scales where greater energy is absorbed.
The insights garnered through adiabatic calorimetry contribute to building a robust foundation of safety measures, ensuring that the hazards identified at the laboratory level are effectively addressed and mitigated during large-scale production using exothermic and endothermic reactions.
The journey of chemical manufacturing is intricately woven with the thread of CRH analysis. From the early identification of products to the optimization of synthetic routes and the challenges of scaling up, a comprehensive understanding of chemical reaction hazards is indispensable. By integrating CRH analysis at each stage, manufacturers not only enhance the safety of their processes but also lay the groundwork for efficient and successful production on a lucrative scale.
Navigating Chemical Stability: Energetic Functional Groups
An aspect that demands early attention is the assessment of energetic functional groups. These groups, embedded within certain classes of chemicals, have a propensity to exhibit heightened reactivity and enthalpy change, particularly under elevated temperatures.
Initial Desktop Work: Unveiling Energetic Functional Groups
When embarking on a new chemical process, whether it involves reagents or the desired products, thorough desktop work becomes imperative. A critical facet of this preliminary exploration is the identification of certain classes of chemicals known to harbour energetic functional groups. These groups are characterised by their potential for increased reactivity, especially when exposed to higher temperatures.
Understanding Nitrogen Groups
One prominent category that frequently raises concerns in terms of stability is nitrogen-containing groups. Nitrogen, while a ubiquitous element, can contribute to the instability of chemicals, particularly when arranged in specific configurations.
Mitigating Risks with Informed Decision-Making
Armed with the knowledge of energetic functional groups, chemists and analysts can make informed decisions during the early stages of process development. Recognising the instability associated with certain groups empowers professionals to choose alternative routes or implement precautionary measures to mitigate potential risks. This proactive approach not only contributes to the safety of the working environment but also aids in devising more robust and efficient chemical processes.
Understanding Scale Differences in Chemical Reactions: Navigating Heat Dynamics
The concept of scale differences influences how processes unfold at various volumes. From millilitres, to litres, to tonnes, the shift in scale introduces significant changes in the relationship between surface area, volume, and heat dynamics.
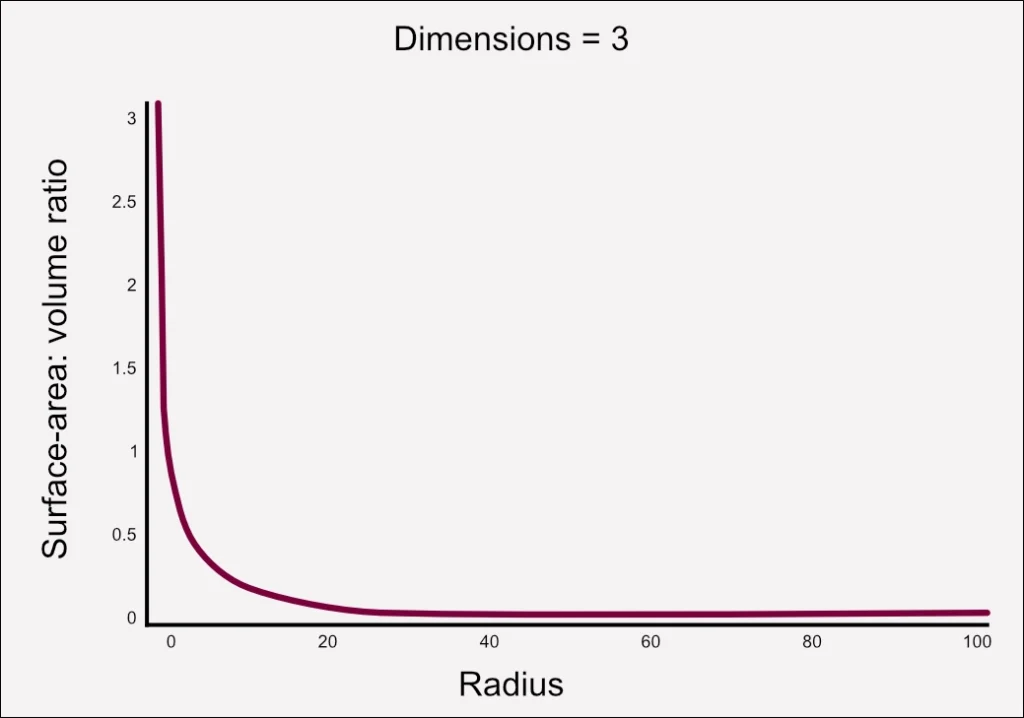
Surface Area and Volume
As the scale of a reactor changes, the relative surface area undergoes a profound transformation. Illustrated by a typical graph for a cylinder, the decrease in radius from right to left results in a steep increase in the surface area-to-volume ratio at smaller vessel sizes. Essentially, as the reactor volume expands, the relative surface area diminishes. This is particularly pertinent to reactor design, given that cooling capacity is intricately linked to the surface area of the reactor.
Cooling Capacity vs. Heat Generation: Imbalance at Scale
Simultaneously, the differences of scale introduce an intriguing dichotomy between cooling capacity and heat generation. While the cooling capacity, represented by the dashed green line in the plot, tends to increase linearly, the heat generation, especially in exothermic processes, follows an exponential trajectory, represented by the red line.
The Arrhenius relationship, a key factor in chemical reactions, dictates that reaction rates increase with temperature. This creates a scenario where the heat generated outpaces the linear increase in cooling capacity.
The Risk of Thermal Runaway: The Intersection
The plot depicts a crucial juncture where the red line of heat generation intersects with the green line of cooling capacity. This intersection signifies a point where the heat produced exceeds the system’s capacity to absorb energy and remove it through normal cooling processes. The consequence is an accumulation of excess heat within the system, leading to a scenario known as thermal runaway.
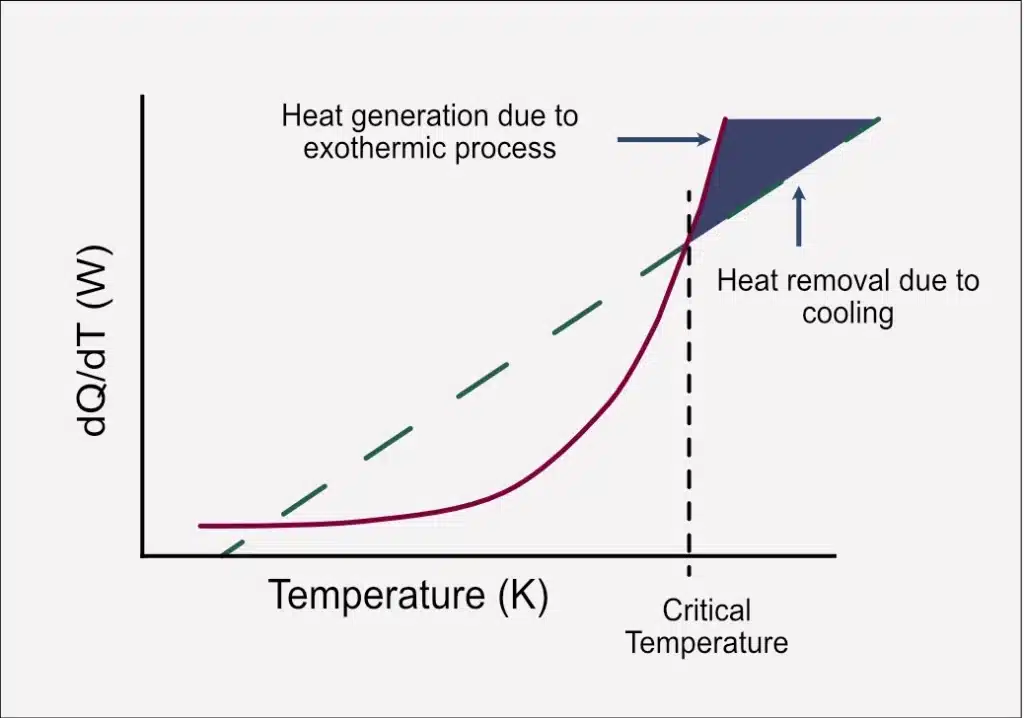
In this situation, the reaction spirals out of control, posing not only safety risks but also potential damage to equipment and the integrity of the overall manufacturing process.
The intricacy between surface area, volume, heat generation, and cooling capacity underscores the need for meticulous planning and design considerations at each scale. By recognising the potential challenges posed by varying reactor sizes, process professionals can foster a secure and controlled environment for chemical syntheses across different scales.
Safeguarding Chemical Processes: Identifying and Mitigating Potential Deviations
The first line of defence against process deviations lies in a comprehensive understanding of the chemistry, energy change, plant infrastructure, and available assessment methods. HAZOP (Hazard and Operability Study), CHAZOP (Computer HAZOP), what-if analysis, Failure Modes and Effects Analysis (FMEA), checklist analysis, and fault tree analysis are among the arsenal of tools used to scrutinise potential deviations.
The Operating Envelope Concept
Central to mitigating process deviations is the concept of designing processes within a safe operating envelope. This involves creating a controlled environment where the operating temperature aligns with the heat generated by potential exothermic reactions.
This operating envelope must also consider the thermal decomposition point of substances within the vessel. Striking this delicate balance allows for the widest possible operating range, enhancing the system’s resilience against unexpected variations.
Aiming for Resilience: Small Changes, Minimal Impact
The ultimate goal in designing chemical processes is to achieve resilience in the face of small changes. Catastrophic outcomes can be averted by meticulously crafting a process that can absorb and adapt to deviations without spiralling out of control.
This necessitates a proactive approach to foresee potential hazards, coupled with the implementation of preventive measures, ensuring that the system can gracefully handle deviations without compromising safety or efficiency.
Safeguarding chemical processes against potential deviations requires a multi-faceted strategy. By combining a profound knowledge of the chemistry and plant infrastructure with rigorous assessment methods, industry professionals can design processes that operate within a safe envelope.
This proactive approach not only minimises the impact of small changes but also fortifies the system against unexpected events, fostering a culture of safety and reliability in the intricate realm of chemical manufacturing.
Navigating the Terrain of Chemical Reaction Hazards
Chemical reaction hazards are unpredictable, but both essential and challenging to assess. As we traverse the complexities of undesired byproducts, pressure fluctuations, and the intricacies of scale, knowledge and analysis is needed to understand the inherent hazards accompanying chemical reactions.
Chemical Reaction Hazard Analysis (CRH) offers a roadmap for manufacturers to navigate the labyrinth of new products, synthetic routes, and the inevitable challenges of scaling up. From early desktop studies to the optimization of synthetic routes, CRH provides a guiding light, allowing professionals to make informed decisions that balance efficiency with safety.
The exploration of Chemical Reaction Hazards must be undertaken with absolute vigilance and there must be a commitment to continuous improvement. Understanding, analysis, and proactive design, will pave the way for safer, more efficient, and more resilient future in the chemical manufacturing industry.